Hydrogen Bonds
by Michael Schmitt
Hydrogen bonds with O-H… N bridges are very important in chemistry and biology. A prototype of O-H… N hydrogen bonded clusters is the PhOH(NH3)n system, which can be studied to obtain a detailed picture of processes like proton transfer and acid-base-reactions, in different electronic states and in the ion.
The simplest aromatic O-H… N hydrogen bonded cluster is the binary PhOH(NH3)1, which has been investigated both experimentally and theoretically by several groups. [1-10]
The R2PI and SHB measurements describred here were carried out with an apparatus described elsewhere in more detail. [11, 12]
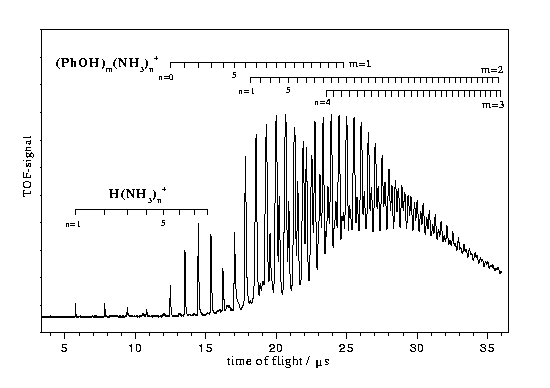
Fig.1 shows a time of flight mass spectrum of a jet-cooled mixture of phenol and ammonia recorded after non-resonant two photon ionization at 285 nm. Ion signal on the mass channels of 1-3 phenol molecules with 0-35 ammonia molecules were recorded. Signals on the mass channels of the fragment ions (NH3)nH+ with n=1-8 could also be observed.
The R2PI spectra recorded on the mass channels of PhOH(NH3)n show narrow bands under one color conditions only for n=1 and 2 [13]. For clusters with n>2 no resonances could be unambigously identified yet by two color R2PI measurements.
However, spectra of good quality of the n³ 2 clusters could be obtained by recording the R2PI signals at the (NH3)nH+ mass (Figure 2). After resonant S1¬ S0 excitation another photon is absorbed to produce an excited ion above the proton transfer barrier. The excess energy can lead to proton transfer followed by dissoziation into a phenoxy radical and (NH3)nH+ or to evaporation of one or more ammonia molecules.
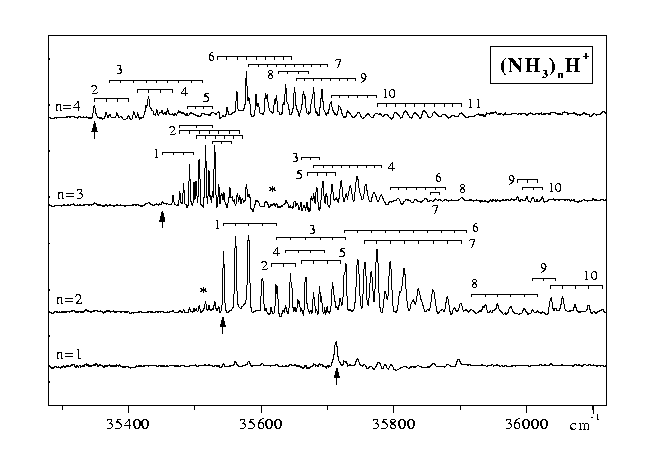
In the first case the excess energy almost quantitatively leads to the separation of fragment ion and phenoxy radical and to very little evaporation of NH3. In the second case the cluster spectra can only be observed on the mass channels of PhOH(NH3)+n£ 2, due to excesive evaporation from the larger clusters and are congested [2, 6]. Both recording the spectra on the fragment ion and on the cluster ion mass channel leads to the vibronic spectrum of the cluster.
[1] N. Mikami, A. Okabe and I. Suzuki, J. Phys. Chem. 92, 1858 (1988)
[2] D. Solgadi, C. Jouvet and A. Tramer, J. Phys. Chem. 92, 3313 (1988)
[3] C. Jouvet, C. Lardeux-Dedonder, M. Richard-Viard, D. Solgadi and A. Tramer, J. Phys. Chem. 94, 5041 (1990)
[4] A. Crepin and A. Tramer, Chem. Phys. 156, 281 (1991)
[5] J. A. Syage and J. Steadman, J. Phys. Chem. 96, 9606 (1992)
[6] A. Schiefke, C. Deusen, C. Jacoby, M. Gerhards, M. Schmitt, K. Kleinermanns and P. Hering,
J. Chem. Phys. 102, 9197 (1995)
[7] J. A. Syage in: Femtosecond Chemistry, ed. J. Manz and L. Wöste, Verlag Chemie, Weinheim (1995)
[8] A. Iwasaki, A. Fujii, T. Watanabe, T. Ebata and N. Mikami, J. Phys. Chem. 100, 16053 (1996)
[9] M. Yi and S. Scheiner, Chem. Phys. Lett. 262, 567 (1996)
[10] W. Siebrand, M. Z. Zgierski, Z. K. Smedarchina, M. Vener and J. Kaneti, Chem. Phys. Lett. 266, 47 (1997)
[11] M. Schmitt, C. Jacoby and K. Kleinermanns, J. Chem. Phys. 108, 4486 (1998)
[12] W. Roth, Ch. Jacoby, A. Westphal and M. Schmitt, J. Phys. Chem. A 102, 3048 (1998)
[13] Ch. Jacoby, P. Hering, M. Schmitt, W. Roth and K. Kleinermanns, Chem. Phys. 239, 23 (1998)
